by Evan Lerner
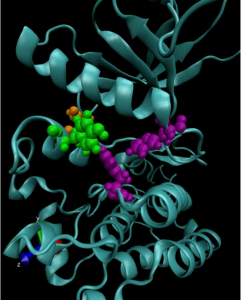
Kinases are a class of enzymes that are responsible for transferring the main chemical energy source used by the body’s cells. As such, they play important roles in diverse cellular processes, including signaling, differentiation, proliferation and metabolism. But since they are so ubiquitous, mutated versions of kinases are frequently found in cancers. Many cancer treatments involve targeting these mutant kinases with specific inhibitors.
Understanding the exact genetic mutations that lead to these aberrant kinases can therefore be critical in predicting the progression of a given patient’s cancer and tailoring the appropriate response.
To achieve this understanding on a more fundamental level, a team of researchers from the University of Pennsylvania’s School of Engineering and Applied Science and Perelman School of Medicine, the Children’s Hospital of Philadelphia (CHOP) and researchers at the Yale School of Medicine’s Cancer Biology Institute, have constructed molecular simulations of a mutant kinase implicated in pediatric neuroblastoma, a childhood cancer impacting the central nervous system.
Using their computational model to study the relationship between single-point changes in the kinase’s underlying gene and the altered structure of the protein it ultimately produces, the researchers revealed useful commonalities in the mutations that result in tumor formation and growth. Their findings suggest that such computational approaches could outperform existing profiling methods for other cancers and lead to more personalized treatments.
The study, published in the Proceedings of the National Academy of Sciences, was led by Ravi Radhakrishnan, Professor and chair of Penn Engineering’s Department of Bioengineering and professor in its Department of Chemical and Biomolecular Engineering, and Mark A. Lemmon, Professor of Pharmacology at Yale and co-director of Yale’s Cancer Biology Institute. The study’s first authors were Keshav Patil, a graduate student in Penn Engineering’s Department of Chemical and Biomolecular Engineering, along with Earl Joseph Jordan and Jin H. Park, then members of the Graduate Group in Biochemistry and Molecular Biology in Penn’s Perelman School of Medicine. Krishna Suresh, an undergraduate student in Radhakrishnan’s lab, Courtney M. Smith, a graduate student in Lemmon’s lab, and Abigail A. Lemmon, an undergraduate in Lemmon’s lab, contributed to the study. They collaborated with Yaël P. Mossé, Associate Professor of Pediatrics at Penn Medicine and in the division of oncology at CHOP.
“Some cancers rely on the aberrant activation of a single gene product for tumor initiation and progression,” says Radhakrishnan. “This unique mutational signature may hold the key to understanding which patients suffer from aggressive forms of the disease or for whom a given therapeutic drug may yield short- or long-term benefits. Yet, outside of a few commonly occurring ‘hotspot’ mutations, experimental studies of clinically observed mutations are not commonly pursued.”
Read the full post in Penn Engineering Today.
