by Nathi Magubane & Ian Scheffler
How does your body distinguish friendly visitors, like medications and medical devices, from dangerous invaders such as viruses and other infectious agents? The answer lies in a protein network dating back half a billion years—before humans diverged from sea urchins, notes Jake Brenner, a physician-scientist at the University of Pennsylvania.
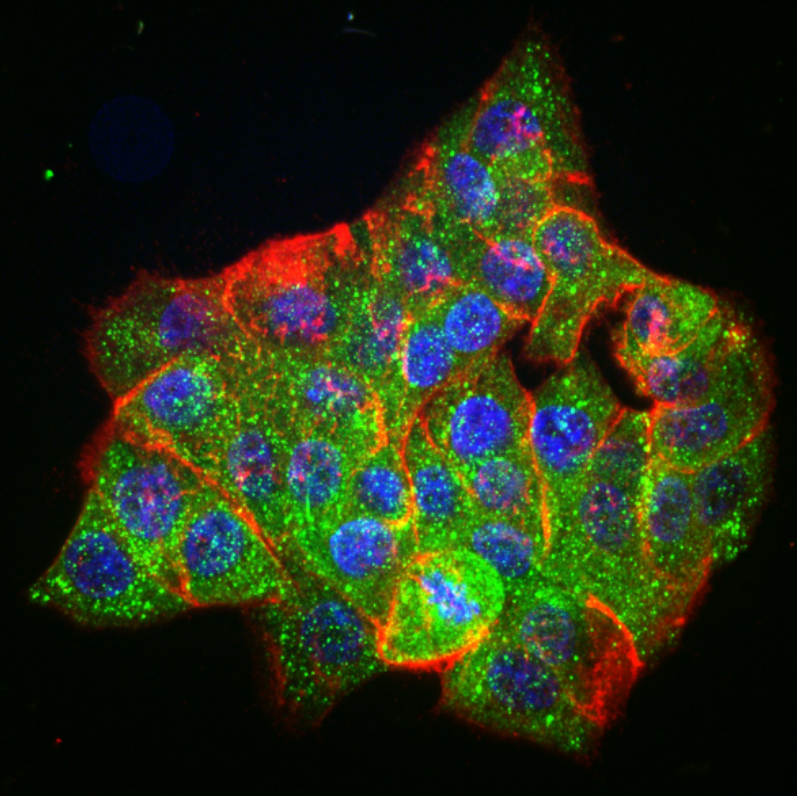
“The complement system is perhaps the oldest-known part of our extracellular immune system,” says Brenner. “It plays a crucial role in identifying foreign materials like microbes, medical devices, or new drugs—particularly the larger ones like in the COVID vaccine.”
The complement system can, however, simultaneously play friend and foe, offering protection with one hand while backhanding the body with the other. In some cases, this ancient network can significantly exacerbate conditions like stroke by targeting the body’s own tissues. As Brenner explains, leaking blood vessels allow complement proteins to target brain tissue, causing the immune system to mistakenly launch an attack on the body’s own cells and worsen patient outcomes.
Now, using a combination of wet-lab experimentation, coupled differential equations, and computational-based modeling and simulations, an interdisciplinary team from the School of Engineering and Applied Science and the Perelman School of Medicine has decrypted the mathematical language behind the complement network’s “decision” to attack.
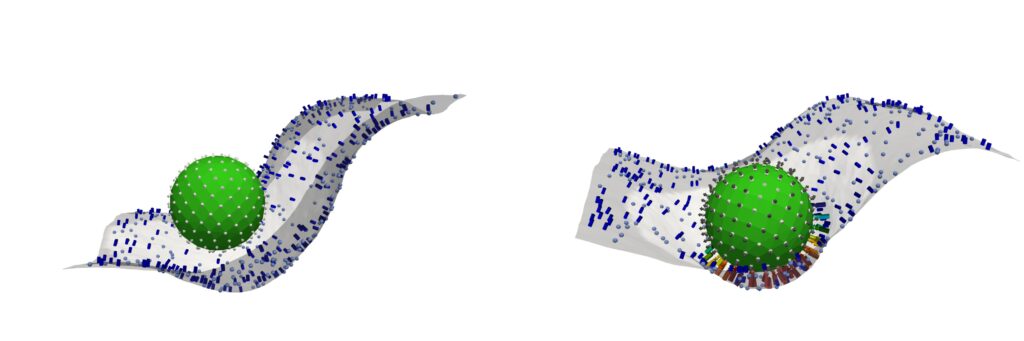
Reporting their findings in Cell, the team identifies a molecular tipping point known as the critical percolation threshold, which is based on how densely complement-binding sites are spaced on the surfaces of the model invader they engineered. If spacing between binding sites is too wide—landing above a threshold—complement activation fizzles out; below it, complement network ignites, a chain reaction of immune agent recruitment which spreads like wildfire.
Read the full story in Penn Today.