by Sophie Burkholder
A New Microscopy Technique Could Reduce the Risk of LASIK Surgery
 Though over ten million Americans have undergone LASIK vision corrective surgery since the option became available about 20 years ago, the procedure still poses some risk to patients. In addition to the usual risks of any surgery however, LASIK has even more due to the lack of a precise way to measure the refractive properties of the eye, which forces surgeons to make approximations in their measurements during the procedure. In an effort to eliminate this risk, a University of Maryland team of researchers in the Optics Biotech Laboratory led by Giuliano Scarcelli, Ph. D., designed a microscopy technique that would allow for precise measurements of these properties.
Though over ten million Americans have undergone LASIK vision corrective surgery since the option became available about 20 years ago, the procedure still poses some risk to patients. In addition to the usual risks of any surgery however, LASIK has even more due to the lack of a precise way to measure the refractive properties of the eye, which forces surgeons to make approximations in their measurements during the procedure. In an effort to eliminate this risk, a University of Maryland team of researchers in the Optics Biotech Laboratory led by Giuliano Scarcelli, Ph. D., designed a microscopy technique that would allow for precise measurements of these properties.
Using a form of light-scattering technology called Brillouin spectroscopy, Scarcelli and his lab found a way to directly determine a patient’s refractive index – the quantity surgeons need to know to be able to measure and adjust the way light travels through the eye. Often used as a way to sense mechanical properties of tissues and cells, this technology holds promise for taking three-dimensional spatial observations of these structures around the eye. Scarcelli hopes to keep improving the resolution of the new technique, to further understanding of the eye, and reduce even more of the risks involved with LASIK surgery.
Taking Tissue Models to the Final Frontier
Space flight is likely to cause deleterious changes to the composition of bacterial flora, leading to an increased risk of infection. The environment may also affect the susceptibility of microorganisms within the spacecraft to antibiotics, key components of flown medical kits, and may modify the virulence of bacteria and other microorganisms that contaminate the fabric of the International Space Station and other flight platforms.
“It has been known since the early days of human space flight that astronauts are more prone to infection,” says Dongeun (Dan) Huh, Wilf Family Term Assistant Professor in Bioengineering at Penn Engineering. “Infections can potentially be a serious threat to astronauts, but we don’t have a good fundamental understanding of how the microgravity environment changes the way our immune system reacts to pathogens.”
In collaboration with G. Scott Worthen, a physician-scientist in neonatology at the Children’s Hospital of Philadelphia, Huh will attempt to answer this question by sending tissues-on-chips to space. Last June, the Center for the Advancement of Science in Space (CASIS) and the National Center for Advancing Translational Sciences (NCATS), part of the National Institutes of Health (NIH), announced that the duo had received funding to study lung host defense in microgravity at the International Space Station.
Huh and Worthen aim to model respiratory infection, which accounts for more than 30 percent of all infections reported in astronauts. The project’s goals are to test engineered systems that model the airway and bone marrow, a critical organ in the immune system responsible for generating white blood cells, and to combine the models to emulate and understand the integrated immune responses of the human respiratory system in microgravity.
Read the rest of the article on Penn Engineering’s Medium Blog. Media contacts Evan Lerner and Janelle Weaver.
Sappi Limited Teams Up with the University of Maine to Develop Paper Microfluidics
At the Westbrook Technology Center of Sappi, a global pulp and paper company, researchers found ways to apply innovations in paper texture for medical use. So far, these include endeavors in medical test devices and patches for patient diagnostics. In collaboration with the Caitlin Howell, Ph.D., Assistant Professor of Chemical and Biomedical Engineering at the University of Maine, Sappi hopes to continue advances in these unconventional uses of their paper, especially as the business in paper for publishing purposes declines.
Sappi’s projects with the university focus on the development of paper microfluidics devices as what’s now becoming a widespread solution for obstacles in point-of-care diagnostics. One project in particular, called Sharklet, uses a paper that mimics shark skin as a way to impede unwanted microbial growth on the device – a key characteristic needed for its transition into commercial use. Beyond this example, Sappi’s work in developing paper microfluidics underscores the benefits of these devices in their mass producibility and adaptability.
New Observations of the WNT Pathway Deepen the Understanding of Protein Signaling in Cellular Development
Scientists at Rice University recently found that a protein signaling pathway called WNT, typically associated with its role in early organism development, can both listen for signals from a large amount of triggers and influence cell types throughout embryonic development. These new findings, published in PNAS, add to the already known functions of WNT, deepening our understanding of it and opening the doors to new potential applications of it in stem cell research.
Led by Aryeh Warmflash, Ph. D., researchers discovered that the WNT pathway is different between stem cells and differentiated cells, contrary to prior belief that it was the same for both. Using CRISPR-Cas9 gene editing technology, the Warmflash lab observed that the WNT signaling pathway is actually context-dependent throughout the process of cellular development. This research brings a whole new understanding to the way the WNT pathway operates, and could open the doors to new forms of gene therapy and treatments for diseases like cancers that involve genetic pathway mutations.
People and Places
In a recent article from Technical.ly Philly, named Group K Diagnostics on a list of ten promising startups in Philadelphia. Group K Diagnostics founder Brianna Wronko graduated with a B.S.E. from Penn’s Department of Bioengineering in 2017, and her point-of-care diagnostics company raised over $2 million in funding last year. Congratulations Brianna!
We would also like to congratulate Pamela K. Woodward, M.D., on her being named as the inaugural Hugh Monroe Wilson Professor of Radiology at the Washington University School of Medicine in St. Louis. Also a Professor of Biomedical Engineering at the university, Dr. Woodward leads a research lab with a focus on cardiovascular imaging, including work on new standards for diagnosis of pulmonary blood clots and on an atherosclerosis imaging agent.
Lastly, we would like to congratulate all of the following researchers on their election to the National Academy of Engineering:
- David Bishop, Ph. D., a professor at the College of Engineering at Boston University whose current research involves the development of personalized heart tissue as an all-encompassing treatment for patients with heart disease.
- Joanna Aizenberg, Ph. D., a professor of chemistry and chemical biology at Harvard University who leads research in the synthesis of biomimetic inorganic materials
- Gilda Barabino, Ph. D., the dean of the City College of New York’s Grove School for Engineering whose lab focuses on cartilage tissue engineering and treatments for sickle cell disease.
- Karl Deisseroth, M.D., Ph. D., a professor of bioengineering at Stanford University whose research involves the re-engineering of brain circuits through novel electromagnetic brain stimulation techniques.
- Rosalind Picard, Ph.D., the founder and director of the Affective Computing Research Group at the Massachusetts Institute of Technology’s Media Lab whose research focuses on the development of technology that can measure and understand human emotion.
- And finally, Molly Stevens, Ph. D., the Research Director for Biomedical Material Sciences at the Imperial College of London with research in understanding biomaterial interfaces for biosensing and regenerative medicine.



 Heart attacks are the result of a stoppage of blood flow to the heart – an interruption to normal function that can result in severe tissue damage, or even tissue death. This loss of healthy tissue function is one of the biggest challenges in treating patients that undergo heart attacks, as the damaged tissue increases their risk of having future attacks. One of the main solutions to this issue right now is the creation of cardiac tissue scaffolds using stem cells to create a platform for new and healthy tissue to grow in vivo. A group of biomedical engineers at Michigan Technological University hopes to
Heart attacks are the result of a stoppage of blood flow to the heart – an interruption to normal function that can result in severe tissue damage, or even tissue death. This loss of healthy tissue function is one of the biggest challenges in treating patients that undergo heart attacks, as the damaged tissue increases their risk of having future attacks. One of the main solutions to this issue right now is the creation of cardiac tissue scaffolds using stem cells to create a platform for new and healthy tissue to grow in vivo. A group of biomedical engineers at Michigan Technological University hopes to 
















 Preeclampsia is potentially life-threatening pregnancy disorder that typically occurs in about 200,000 expectant mothers every year. With symptoms of high blood pressure, swelling of the hands and feet, and protein presence in urine, preeclampsia is usually treatable if diagnosed early enough. However, current methods for diagnosis involve invasive procedures like cordocentesis, a procedure which takes a sample of fetal blood.
Preeclampsia is potentially life-threatening pregnancy disorder that typically occurs in about 200,000 expectant mothers every year. With symptoms of high blood pressure, swelling of the hands and feet, and protein presence in urine, preeclampsia is usually treatable if diagnosed early enough. However, current methods for diagnosis involve invasive procedures like cordocentesis, a procedure which takes a sample of fetal blood.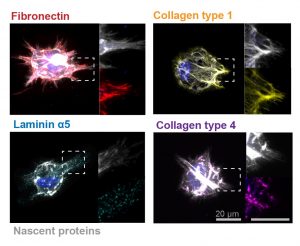
 Though over ten million Americans have undergone LASIK vision corrective surgery since the option became available about 20 years ago, the procedure still poses some risk to patients. In addition to the usual risks of any surgery however, LASIK has even more due to the lack of a precise way to measure the refractive properties of the eye, which forces surgeons to make approximations in their measurements during the procedure. In an effort to eliminate this risk, a University of Maryland team of researchers in the Optics Biotech Laboratory led by
Though over ten million Americans have undergone LASIK vision corrective surgery since the option became available about 20 years ago, the procedure still poses some risk to patients. In addition to the usual risks of any surgery however, LASIK has even more due to the lack of a precise way to measure the refractive properties of the eye, which forces surgeons to make approximations in their measurements during the procedure. In an effort to eliminate this risk, a University of Maryland team of researchers in the Optics Biotech Laboratory led by