by Win Reynolds
 A research team led by engineers at the University of Pennsylvania and Northwestern University scientists has created a new synthetic biology approach, or a “QR code for cancer cells,” to follow tumor cells over time, finding there are meaningful differences in why a cancer cell dies or survives in response to anti-cancer therapies.
A research team led by engineers at the University of Pennsylvania and Northwestern University scientists has created a new synthetic biology approach, or a “QR code for cancer cells,” to follow tumor cells over time, finding there are meaningful differences in why a cancer cell dies or survives in response to anti-cancer therapies.
Remarkably, what fate cancer cells choose after months of therapy is “entirely predictable” based on seemingly small, yet important, differences that appear even before treatment begins. The researchers also discovered the reason is not genetics, contrary to beliefs held in the field.
The findings were recently published in Nature.
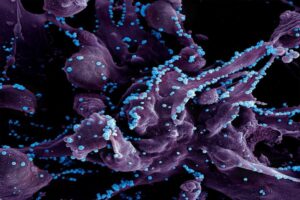
The study outlined the team’s new technology platform that developed a QR code for each of the millions of cells for scientists to find and use later — much like tagging swans in a pond. The QR code directs researchers to a genome-wide molecular makeup of these cells and provides information about how they’ve reacted to cancer treatment.
“We think this work stands to really change how we think about therapy resistance,” said Arjun Raj, co-senior author and Professor in Bioengineering in the School of Engineering and Applied Science at the University of Pennsylvania. “Rather than drug-resistant cells coming in just one flavor, we show that even in highly controlled conditions, different ‘flavors’ can emerge, raising the possibility that each of these flavors may need to be treated individually.”
In the study, the lab and collaborators sought to apply synthetic biology tools to answer a key question in cancer research: What makes certain tumors come back a few months or years after therapy? In other words, could the lab understand what causes some rare cells to develop therapeutic resistance to a drug?
“There are many ways cells become different from each other,” said Yogesh Goyal, the co-senior author at Northwestern University. “Our lab asks, how do individual cells make decisions? Understanding this in the context of cancer is all the more exciting because there’s a clinically relevant dichotomy: A cell dies or becomes resistant when faced with therapies.”
Using the interdisciplinary team, the scientists put the before-and-after cloned cells through a whole genome sequencing pipeline to compare the populations and found no systematic underlying genetic mutations to investigate the hypothesis. Raj and Goyal helped develop the QR code framework, FateMap, that could identify each unique cell that seemed to develop resistance to drug therapy. “Fate” refers to whether a cell dies or survives (and if so, how), and the scientists “map” the cells across their lifespan, prior to and following anti-cancer therapy. FateMap is the result of work from several research institutions, and it applies an amalgamation of concepts spanning several disciplines, including synthetic biology, genome engineering, bioinformatics, machine learning and thermodynamics.
“Some are different by chance — just as not all leaves on a tree look the same — but we wanted to determine if that matters,” Goyal said. “The cell biology field has a hard time defining if differences have meaning.”
Read the full story in Penn Engineering Today.



 Carlos Armando Aguila, Ph.D. student in Bioengineering, is a member of the Center of Neuroengineering and Therapeutics, advised by
Carlos Armando Aguila, Ph.D. student in Bioengineering, is a member of the Center of Neuroengineering and Therapeutics, advised by  Joseph Lance Victoria Casila is a Ph.D. student in Bioengineering in the lab of
Joseph Lance Victoria Casila is a Ph.D. student in Bioengineering in the lab of  Trevor Chan is a Ph.D. student in Bioengineering in the lab of
Trevor Chan is a Ph.D. student in Bioengineering in the lab of  Rakan El-Mayta is an incoming Ph.D. student in the lab of
Rakan El-Mayta is an incoming Ph.D. student in the lab of  Austin Jenk is a Ph.D. student in the lab of
Austin Jenk is a Ph.D. student in the lab of  Jiageng Liu is a Ph.D. student in the lab of
Jiageng Liu is a Ph.D. student in the lab of  Alexandra Neeser is a Ph.D. student in the lab of
Alexandra Neeser is a Ph.D. student in the lab of  William Karl Selboe Ojemann, a Ph.D. Student in Bioengineering, is a member of the Center for Neuroengineering and Therapeutics directed by
William Karl Selboe Ojemann, a Ph.D. Student in Bioengineering, is a member of the Center for Neuroengineering and Therapeutics directed by  Savan Patel (BSE Class of 2023) conducted research in the lab of
Savan Patel (BSE Class of 2023) conducted research in the lab of  David E. Reynolds, a Ph.D. student in Bioengineering, is a member of the lab of
David E. Reynolds, a Ph.D. student in Bioengineering, is a member of the lab of  Andre Roots is a Ph.D. student in the lab of
Andre Roots is a Ph.D. student in the lab of  Emily Sharp, a second year Ph.D. student in Bioengineering, is a member of the lab of
Emily Sharp, a second year Ph.D. student in Bioengineering, is a member of the lab of  Nat Thurlow is a Ph.D. student in the lab of
Nat Thurlow is a Ph.D. student in the lab of  Maggie Wagner, Ph.D. student in Bioengineering, is a member in the labs of
Maggie Wagner, Ph.D. student in Bioengineering, is a member in the labs of