To combat the COVID-19 pandemic caused by the SARS-CoV2 virus, Dr. Andrew Tsourkas’s Targeted Imaging Therapeutics and Nanomedicine (Titan) Lab in Penn Bioengineering, in collaboration with the Penn-based startup, AlphaThera, was recently awarded a $667,000 SBIR Phase II Grant Extension to support its efforts in commercializing COVID-19 detection technology. The grant supports work to address the growing need for anti-viral antibody testing. Specifically, the Tsourkas Lab and AlphaThera hope to leverage their expertise with antibody conjugation technologies to reduce the steps and complexity of existing detection assays to enable greater production and higher sensitivity tests. AlphaThera was founded in 2016 by Andrew Tsourkas, PhD, Professor of Bioengineering and James Hui, MD, PhD, a graduate of the Perelman School of Medicine and Penn Bioengineering’s doctoral program.
During this pandemic it is crucial to characterize disease prevalence among populations, understand immunity, test vaccine efficacy and monitor disease resurgence. Projections have indicated that millions of daily tests will be needed to effectively control the virus spread. One important testing method is the serological assay: These tests detect the presence of SARS-CoV2 antibodies in a person’s blood produced by the body’s immune system responding to infection. Serological tests not only diagnose active infections, but also establish prior infection in an individual, which can greatly aid in forecasting disease spread and contact tracing. To perform the serological assays for antibody detection, well-established immunoassay methods are used such as ELISA.
A variety of issues have slowed the distribution of these serological assays for antibody testing. The surge in demand for testing has caused shortages in materials and reagents that are crucial for the assays. Furthermore, complexity in some of the assay formats can slow both production and affect the sensitivity of test results. Recognizing these problems, AlphaThera is leveraging its novel conjugation technology to greatly improve upon traditional assay formats.
With AlphaThera’s conjugation technology, the orientation of antibodies can be precisely controlled so that they are aligned and uniformly immobilized on assay detection plates. This is crucial as traditional serological assays often bind antibodies to plates in a non-uniform manner, which increases variability of results and reduces sensitivity. See Fig 1 below. With AlphaThera’s uniform antibody immobilization, assay specificity could increase by as much as 1000- fold for detection of a patient’s SaRS-CoV2 antibodies.
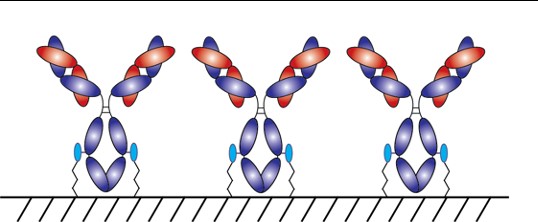
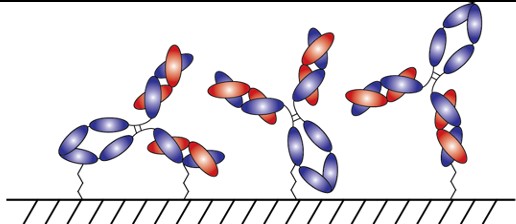
Furthermore, AlphaThera is addressing the shortage of assay reagents, specifically secondary antibody reagents, by removing certain steps from traditional serological assays. Rather than relying on secondary antibodies for detection of the patient antibodies, AlphaThera’s technology can label the patient SaRS-CoV2 primary antibodies directly in serum with a detection reagent. This eliminates several processing steps, reducing the time of the assay by as much as 50%, as well as the costs.
The Tsourkas Lab and AlphaThera have initiated their COVID-19 project, expanding into the Pennovation Center and onboarding new lab staff. Other antibody labeling products have also become available and are currently being prepared for commercialization. Check out the AlphaThera website to learn more about their technology at https://www.alphathera.com.
NIH SBIR Phase II Grant Extension— 5-R44-EB023750-03 (PI: Yu) — 10/07/2020 – 10/07/2021
