A Closer Look
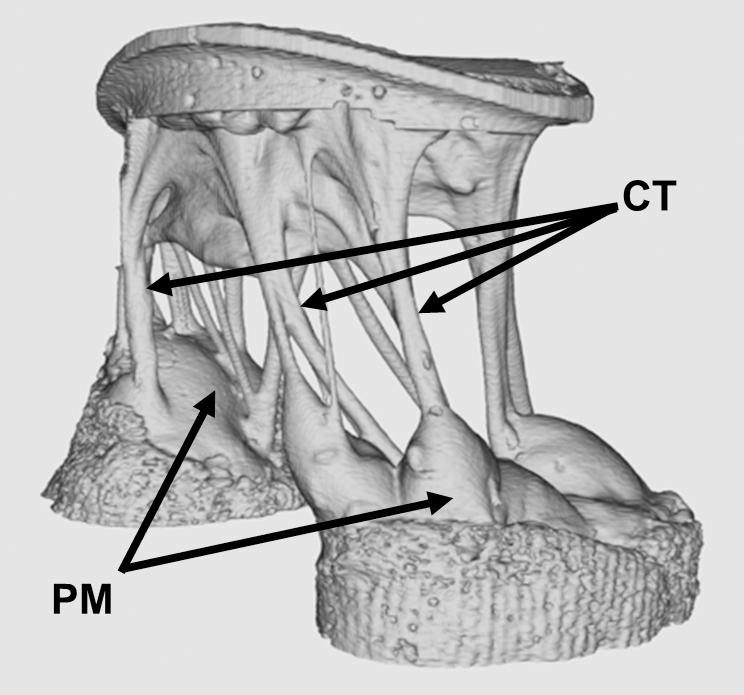
Since its invention in the early 1970s, magnetic resonance imaging (MRI) has played an increasingly important role in the diagnosis of illness. In addition, over time, the technology of MRI has evolved enormously, with the ability to render more detailed three-dimensional images using stronger magnetic fields . However, imaging tissues under mechanical loads (e.g., beating heart, lung breathing) are still difficult to image precisely with MRI.
A new study in PLOS One, led by Morten Jensen, Ph.D., of the University of Arkansas, breaks an important technical barrier in high resolution imaging for tissues under mechanical load. Using 3D-printed mounting hardware and 7-tesla MRI, this group produced some of the highest quality images yet produced of the mitral valve exposed to physiological pressures (see above). In the longer term, this method could point to new corrective surgical procedures that would greatly improve the repair procedures for mitral valves.
Oncology Breakthroughs
Among the most significant challenges faced by surgical oncologists is developing a ‘clear margin,’ meaning that the tissue remaining after tumor excision is free of any tumor cells. If the margins are not free of tumor cells, the cancer is more likely to recur. However, until now, it has been impossible to determine if cancer cells were still present in tissue margins before finishing surgery because of the time required to test specimens.
At the University of Texas, Austin, however, scientists are getting closer to overcoming this obstacle. In a recent study published in Science Translational Medicine, this team of scientists presents the MasSpecPen — short for mass spectrometry pen. This device is capable of injecting a tiny drop of water into tissue, extracting the water after it mixes with the tissue, and quickly analyzing the sample’s molecular components. The authors, who included biomedical engineering faculty member Thomas E. Milner, Ph.D., tested the device using ex vivo samples from 253 patients with different varieties of cancer, including breast, lung, and thyroid cancers. The MasSpecPen provided sensitivity, specificity, and accuracy exceeding 96% in all cases. Although it has yet to be tested intraoperatively, if effective under those conditions, the device could become an essential part of the surgeon’s arsenal.
If the MasSpecPen could render surgical treatment of cancer more effective, a device developed at SUNY Buffalo could help doctors diagnose lung cancer earlier. Lung cancer is a particularly deadly variety of cancer because patients don’t feel any discomfort until the cancer has spread to other areas of the body. In collaboration with Buffalo’s Roswell Park Center Institute, microchip manufacturer Intel, and local startup Garwood Medical Devices, a team of scientists, including Professor Edward P. Furlani, Ph.D., from Buffalo’s Department of Chemical and Biomedical Engineering, was awarded a grant from the National Science Foundation to develop a subcutaneous implant incorporating a nanoplasmonic biochip to detect biomarkers of lung cancer. A wearable smart band would receive data from the biochip and would act as an early warning system for lung cancer. The biomarkers selected for the biochip would optimally predict lung cancer risk much earlier than the metastasis stage. If the system that the team develops is successful in diagnosing lung cancer before it spreads, it could greatly improve survival and cure rates.
Feverish Growth
Worldwide but particularly in the Global South, malaria remains a major public health concern. According to a Global Burden of Disease study in 2015, there were nearly 300 million cases of the disease in a single year, with 731,000 fatalities. One of the earliest treatments to combat malaria was invented by British colonialists, who added quinine to the tonic used in the gin and tonic cocktail. More recently the drug artemisinin was developed for fighting malaria. However, this drug and its derivatives are very expensive. The primary reason for this cost is that the drug is extracted from the sweet wormwood plant, which is in short supply. In hopes of producing a greater supply of artemisinin, scientists collaborating among Denmark, Malaysia, and the Netherlands report in Frontiers in Bioengineering and Biotechnology that transplanting the genes responsible for producing atremisinin into Physcomitrella patens, a common moss, led to a much faster production rate of the drug than what is possible with the wormwood plant. The process proved simpler and less expensive than earlier attempts to transplant genes into tobacco plants. If this potential is harnessed correctly, it could make an enormous difference in lessening the global burden of malaria.
Understanding Fear
We’ve known for years about the flight-or-fight response — the adrenergic response of our bodies to danger, which we share in common with a number of other animals. Once the decision to flee is made, however, we know far less about what determines the escape strategy used. According to Malcolm A. MacIver, professor of biomedical engineering and mechanical engineering in Northwestern University’s McCormick School of Engineering, part of the escape strategy depends on how far away the attacker is. In a paper he coauthored that was published in Current Biology, Dr. MacIver studied threat responses in larval zebrafish and found that a fast-looming stimulus produced either freezing or escape at a shorter interval following the threat perception; when the perceived threat was slow looming, longer latency following the perception of the threat was seen, resulting in a greater variety of types of escape behaviors. While it might seem a giant leap between observing behaviors in fish and higher life forms, the basic mechanism in the “oldest” parts of the brain, from an evolutionary standpoint, are less different than we might think.
People and Places
The University of Maryland has announced that construction on a new building to serve as the home of its Department of Bioengineering will be finished by the end of September. The building is to be named A. James Clark Hall, after a builder, philanthropist, and alumnus of Maryland’s School of Engineering. Further south, George Mason University in Fairfax, Va., has announced that the new chair of its Department of Engineering there will be Michael Buschmann, Ph.D., an alumnus of MIT and faculty member since the 1990s at École Polytechnique in Montreal. Congratulations, Dr. Buschmann!
