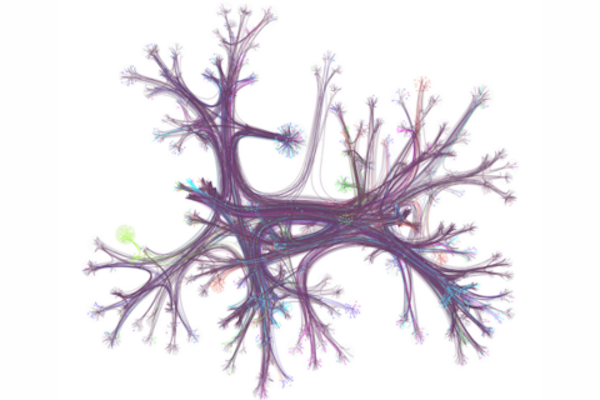
At one point or another, you may have gone online looking for a specific bit of information and found yourself “going down the Wiki rabbit hole” as you discover wholly new, ever-more fascinating related topics — some trivial, some relevant — and you may have gone so far down the hole it’s difficult to piece together what brought you there to begin with.
According to the University of Pennsylvania’s Dani Bassett, who recently worked with a collaborative team of researcher to examine the browsing habits of 482,760 Wikipedia readers from 50 different countries, this style of information acquisition is called the “busybody.” This is someone who goes from one idea or piece of information to another, and the two pieces may not relate to each other much.
“The busybody loves any and all kinds of newness, they’re happy to jump from here to there, with seemingly no rhyme or reason, and this is contrasted by the ‘hunter,’ which is a more goal-oriented, focused person who seeks to solve a problem, find a missing factor, or fill out a model of the world,” says Bassett.
In the research, published in the journal Science Advances, Bassett and colleagues discovered stark differences in browsing habits between countries with more education and gender equality versus less equality, raising key questions about the impact of culture on curiosity and learning.
Read the full story in Penn Today.
Dani S. Bassett is the J. Peter Skirkanich Professor at the University of Pennsylvania with a primary appointment in the School of Engineering and Applied Science’s Department of Bioengineering and secondary appointments in the School of Arts & Sciences’ Department of Physics & Astronomy, Penn Engineering’s Department of Electrical and Systems Engineering, and the Perelman School of Medicine’s Departments of Neurology and Psychiatry.





 Carlos Armando Aguila, Ph.D. student in Bioengineering, is a member of the Center of Neuroengineering and Therapeutics, advised by
Carlos Armando Aguila, Ph.D. student in Bioengineering, is a member of the Center of Neuroengineering and Therapeutics, advised by  Joseph Lance Victoria Casila is a Ph.D. student in Bioengineering in the lab of
Joseph Lance Victoria Casila is a Ph.D. student in Bioengineering in the lab of  Trevor Chan is a Ph.D. student in Bioengineering in the lab of
Trevor Chan is a Ph.D. student in Bioengineering in the lab of  Rakan El-Mayta is an incoming Ph.D. student in the lab of
Rakan El-Mayta is an incoming Ph.D. student in the lab of  Austin Jenk is a Ph.D. student in the lab of
Austin Jenk is a Ph.D. student in the lab of  Jiageng Liu is a Ph.D. student in the lab of
Jiageng Liu is a Ph.D. student in the lab of  Alexandra Neeser is a Ph.D. student in the lab of
Alexandra Neeser is a Ph.D. student in the lab of  William Karl Selboe Ojemann, a Ph.D. Student in Bioengineering, is a member of the Center for Neuroengineering and Therapeutics directed by
William Karl Selboe Ojemann, a Ph.D. Student in Bioengineering, is a member of the Center for Neuroengineering and Therapeutics directed by  Savan Patel (BSE Class of 2023) conducted research in the lab of
Savan Patel (BSE Class of 2023) conducted research in the lab of  David E. Reynolds, a Ph.D. student in Bioengineering, is a member of the lab of
David E. Reynolds, a Ph.D. student in Bioengineering, is a member of the lab of  Andre Roots is a Ph.D. student in the lab of
Andre Roots is a Ph.D. student in the lab of  Emily Sharp, a second year Ph.D. student in Bioengineering, is a member of the lab of
Emily Sharp, a second year Ph.D. student in Bioengineering, is a member of the lab of  Nat Thurlow is a Ph.D. student in the lab of
Nat Thurlow is a Ph.D. student in the lab of  Maggie Wagner, Ph.D. student in Bioengineering, is a member in the labs of
Maggie Wagner, Ph.D. student in Bioengineering, is a member in the labs of 
