by

How do you make robotics kits affordable for children in low-income countries? Speed up the manufacturing of organs-on-a-chip? Lower the environmental impact of condiments in restaurants?
If you’re a senior at Penn Engineering, the answer is to team up with your peers in the Senior Design Project Competition, which every year draws interdisciplinary groups from across the School’s six majors to solve real-world problems. Championed by the late Walter Korn (EE’57, GEE’68), a past president of the Engineering Alumni Society (EAS), Senior Design also invites alumni back to campus to evaluate the seniors’ year-long capstone projects.
Since the program started nearly two decades ago, hundreds of alumni have shared centuries’ worth of their collective experience with soon-to-be-minted graduates in the form of constructive feedback. “Senior Design is really one of the best days at Penn Engineering,” says Bradley Richards (C’92, LPS’17), Director of Alumni Relations, who manages the program. “Faculty advisors work with students all year long to bring out the best in each group’s efforts, and the results speak for themselves.”
This year, three student teams from each of Penn Engineering’s six departments — Bioengineering (BE), Chemical and Biomolecular Engineering (CBE), Computer and Information Science (CIS), Electrical and Systems Engineering (ESE), Materials Science and Engineering (MSE), and Mechanical Engineering and Applied Mechanics (MEAM) — presented their work to more than 60 alumni in person and online.
Judges’ Choice Award
The Judges’ Choice Award, which recognizes overall excellence, went to ESE’s VivoDisk, which developed a novel machine to manufacture organs-on-a-chip for Vivodyne, a startup launched by Dan Huh, Associate Professor in BE.
As one of the team members, Akash Chauhan (ENG’24), learned while interning for Vivodyne, assembling the stacks of organs-on-a-chip, which are collections of plastic plates containing cells that simulate organs for preclinical drug testing, is extremely finicky and time consuming.
By developing a machine that could automatically align the plates with high precision using computer vision and AI, the team reduced the disks’ manufacturing time and expense, leading Vivodyne to adopt the device for commercial use, accelerating the process of drug discovery. VivoDisk’s team members included Chauhan; Angela Rodriguez (ENG’24), Aliris Tang (ENG’24, W’24), Dagny Lott (ENG’24), Simone Kwee (ENG’24) and Vraj Satashia (ENG’24, GEN’25) and was advised by Sid Deliwala, Alfred Moore Senior Fellow and Director of Lab Programs in ESE, and Jan Van der Spiegel, Professor in ESE.
Technology and Innovation Award
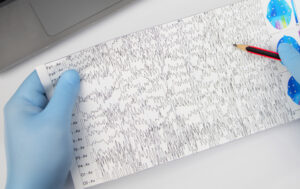
One of the greatest challenges for children with epilepsy is status epilepticus, an abnormal type of long-lasting seizure that is hard to distinguish from typical seizures and that has a mortality rate of 30%. There is currently no way to perform a test for status epilepticus at home, meaning that children suspected of having the condition must be rushed to the hospital for an electroencephalogram.
Epilog, a team from BE, developed a novel, wearable headset that analyzes brainwaves to accurately determine whether or not a child suffering a seizure is actually suffering from status epilepticus. The team, composed of Rohan Chhaya (ENG’24, GEN’24), Carly Flynn (ENG’24), Elena Grajales (ENG’24), Priya Shah (ENG’24, GEN’25) and Doris Xu (ENG’24) and advised by Erin Berlew, Research Scientist in the Department of Orthopaedic Surgery and Lecturer in BE, carefully validated the device’s accuracy.
The judges recognized Epilog’s technological expertise, which ran the gamut from software to hardware, including a custom app to work with the device and carefully considered features like electrodes whose position can be adjusted to accommodate a child’s growth over time.
Read the full story in Penn Engineering Today.