
To treat large gaps in long bones, like the femur, which result from bone tumor removal or a shattering trauma, researchers at Penn Medicine and the University of Illinois at Chicago developed a process that partially recreates the bone growth process that occurs before birth. A bone defect of more than two centimeters is considered substantial, and current successful healing rates stand at 50% or less, with failure often resulting in amputation. The team hopes that their method, which they’ve developed in rodent models to mimic the process of rapid fetal bone growth, can substantially improve success rates. Their findings are published in Science Translational Medicine.
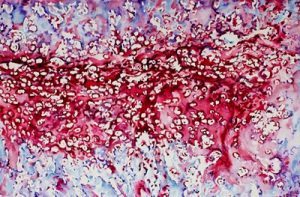
“When bones are originally formed in the embryo, they’re first generated from cartilage, like a template,” says senior author Joel Boerckel, an assistant professor of orthopaedic surgery and bioengineering. “In order to regenerate bone within defects that otherwise won’t heal in grown people, we are seeking to recreate the embryonic bone development process.”
To do that, the researchers’ process begins with the delivery of specially engineered stem cells (called a condensation of mesenchymal cells) to the rodents’ bone defect, which sparks endochondral ossification, the specific term for embryonic bone development.
Read more at Penn Medicine News.
