While modern cancer treatments can have tremendous therapeutic impact, formidable obstacles remain. Foremost among these is drug resistance, the ability of cancers to withstand and ultimately progress despite the presence of an anti-cancer drug. However, ongoing research provides hope that these challenges can be overcome, including recent work performed by Penn Engineers.
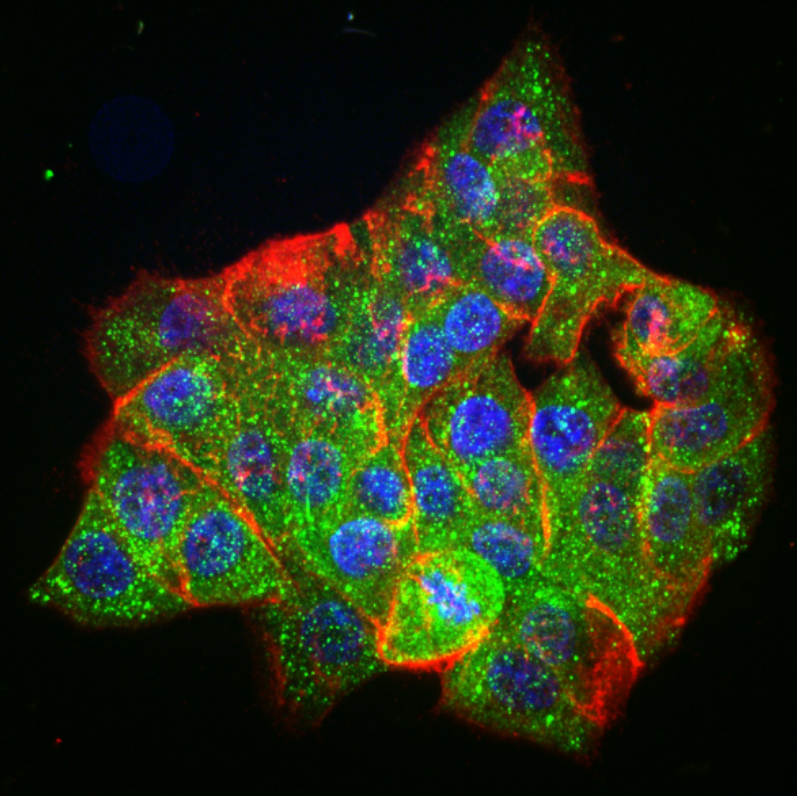
The lab of Lukasz J. Bugaj, Assistant Professor in the Department of Bioengineering, recently published an article that uncovers new mechanisms of how oncogenes interact with important pathways of cellular signaling that are associated with resistance. This work, titled “Oncogenic EML4-ALK Assemblies Suppress Growth Factor Perception and Modulate Drug Tolerance,” applied a new technique called ‘optogenetic functional profiling’ that allowed measurement of how important molecular signaling pathways respond to precise perturbations applied by the researchers. By applying this technique to many different cell types, the group found important differences in resistance-associated signaling between cancer cells and healthy cells
Specifically, the research showed that an oncogene called EML4-ALK, which activates oncogenic signaling, simultaneously inactivates adjacent pathways that can cause resistance. As a consequence, once an oncogene-blocking drug is applied, the inactivation is relieved, thus boosting activity through these adjacent, resistance-associated pathways. The study also showed that these pathways were not only de-repressed, but were actively stimulated by neighboring cancer cells, further enhancing cell survival in the presence of the drug.
“Our work shows that oncogenes, while driving cell division in cancer cells, simultaneously suppress the cells’ regulation by their environment,” said Dr. Bugaj. “While the work reveals mechanisms of paradoxical responses to drug treatment related to resistance, they may also inspire new ideas for therapies that can more efficiently kill cancer cells while maintaining suppression of resistance signaling. This work was co-led by PhD student David Gonzalez-Martinez and by Lee Roth, PhD, a postdoctoral fellow, and was supported by a grant from the American Cancer Society.
Dr. Bugaj’s article can be read here.