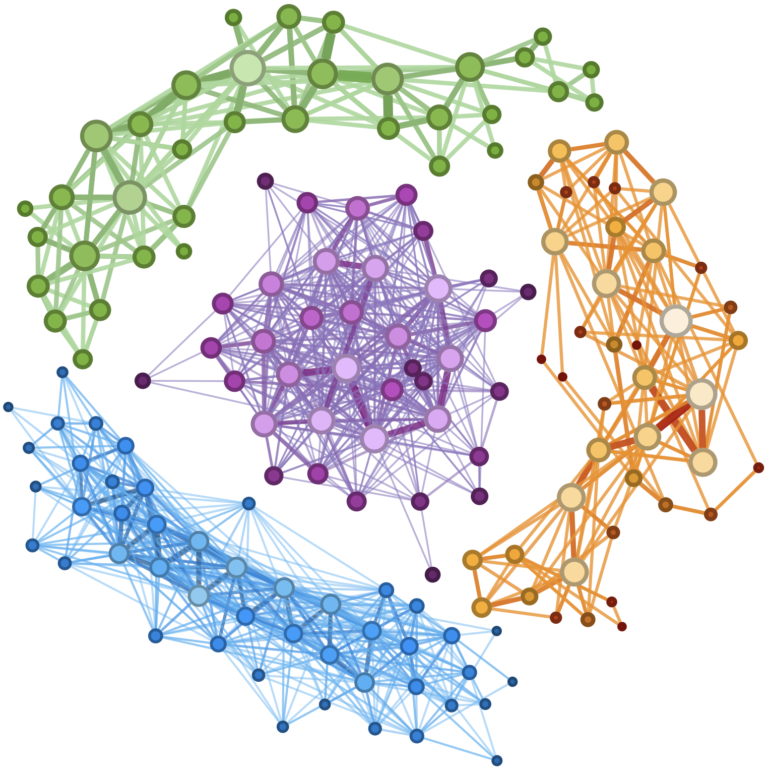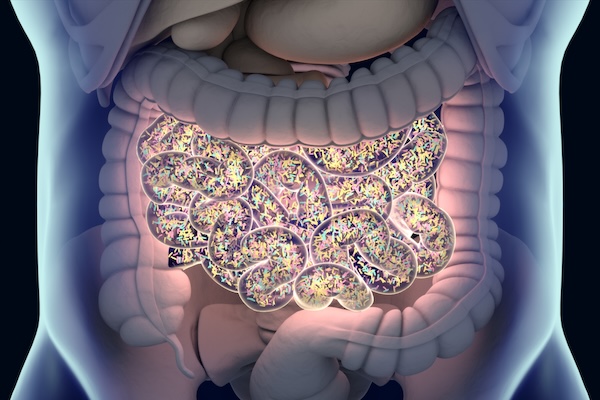
The average human gut contains roughly 100 trillion microbes, many of which are constantly competing for limited resources. “It’s such a harsh environment,” says César de la Fuente, Presidential Assistant Professor in Bioengineering and in Chemical and Biomolecular Engineering within the School of Engineering and Applied Science, in Psychiatry and Microbiology within the Perelman School of Medicine, and in Chemistry within the School of Arts & Sciences. “You have all these bacteria coexisting, but also fighting each other. Such an environment may foster innovation.”
In that conflict, de la Fuente’s lab sees potential for new antibiotics, which may one day contribute to humanity’s own defensive stockpile against drug-resistant bacteria. After all, if the bacteria in the human gut have to develop new tools in the fight against one another to survive, why not use their own weapons against them?
In a new paper in Cell, the labs of de la Fuente and Ami S. Bhatt, Professor in Medicine (Hematology) and Genetics at Stanford, surveyed the gut microbiomes of nearly 2,000 people, discovering dozens of potential new antibiotics. “We think of biology as an information source,” says de la Fuente. “Everything is just code. And if we can come up with algorithms that can sort through that code, we can dramatically accelerate antibiotic discovery.”
Read the full story in Penn Engineering Today.