by Kelsey Geesler

Important brain structures that are key for signaling in the brain are narrower and less dense in females, and more likely to be damaged by brain injuries, such as concussion. Long-term cognitive deficits occur when the signals between brain structures weaken due to the injury. The structural differences in male and female brains might explain why females are more prone to concussions and experience longer recovery from the injury than their male counterparts, according to a preclinical study led by the Perelman School of Medicine at the University of Pennsylvania, published this week in Acta Neuropathologica.
Each year, approximately 50 million individuals worldwide suffer a concussion, also referred to as mild traumatic brain injury (TBI). However, there is nothing “mild” about this condition for the more than 15 percent of individuals who suffer persisting cognitive dysfunction, which includes difficulty concentrating, learning and remembering new information, and making decisions.
Although males make up the majority of emergency department visits for concussion, this has been primarily attributed to their greater exposure to activities with a risk of head impacts compared to females. In contrast, it has recently been observed that female athletes have a higher rate of concussion and appear to have worse outcomes than their male counterparts participating in the same sport.
“Clinicians have observed for a long time that females suffer from concussion at higher rates than males in the same sports, and that they take longer to recover cognitive function, but couldn’t explain the underlying mechanisms of this phenomenon,” said senior author Douglas Smith, MD, a professor of Neurosurgery and director of Penn’s Center for Brain Injury and Repair. “The variances in brain structures of females and males not only illuminate why this disparity exists, but also exposes biomarkers, such as axon protein fragments, that can be measured in the blood to determine injury severity, monitor recovery, and eventually help identify and develop treatments that help patients repair these damaged structures and restore cognitive function.”
Read the full story in Penn Medicine News.
Douglas H. Smith is a member of the Penn Bioengineering Graduate Group.




 Speaker:
Speaker: 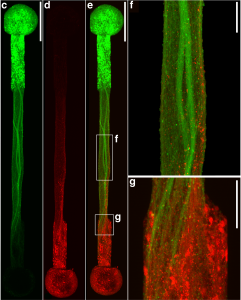


 Penn’s brainSTIM center will study neuromodulation to repair and enhance human brain functio
Penn’s brainSTIM center will study neuromodulation to repair and enhance human brain functio