“We need to think big in antibiotics research,” says Cesar de la Fuente. “Over one million people die every year from drug-resistant infections, and this is predicted to reach 10 million by 2050. There hasn’t been a truly new class of antibiotics in decades, and there are so few of us tackling this issue that we need to be thinking about more than just new drugs. We need new frameworks.”
De la Fuente is Presidential Assistant Professor in the Department of Bioengineering and the Department of Chemical and Biomolecular Engineering at the University of Pennsylvania School of Engineering and Applied Science. He holds additional primary appointments in Psychiatry and Microbiology in the Perelman School of Medicine.
De la Fuente’s lab, the Machine Biology Group, creates these new frameworks using potent partnerships in engineering and the health sciences, drawing on the “power of machines to accelerate discoveries in biology and medicine.”
Marrying artificial intelligence with advanced experimental methods, the group has mined the ancient past for future medical breakthroughs. In a recent study published in Cell Host and Microbe, the team has launched the field of “molecular de-extinction.”
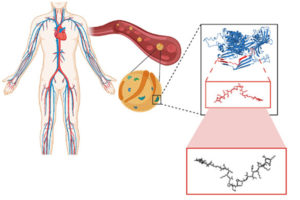
Our genomes – our genetic material – and the genomes of our ancient ancestors, express proteins with natural antimicrobial properties. “Molecular de-extinction” hypothesizes that these molecules could be prime candidates for safe new drugs. Naturally produced and selected through evolution, these molecules offer promising advantages over molecular discovery using AI alone.
In this paper, the team explored the proteomic expressions of two extinct organisms –Neanderthals and Denisovans, archaic precursors to the human species – and found dozens of small protein sequences with antibiotic qualities. Their lab then worked to synthesize these molecules, bringing these long-since-vanished chemistries back to life.
“The computer gives us a sequence of amino acids,” says de la Fuente. “These are the building blocks of a peptide, a small protein. Then we can make these molecules using a method called ‘solid-phase chemical synthesis.’ We translate the recipe of amino acids into an actual molecule and then build it.”
The team next applied these molecules to pathogens in a dish and in mice to test the veracity and efficacy of their computational predictions.
“The ones that worked, worked quite well,” continues de la Fuente. “In two cases, the peptides were comparable – if not better – than the standard of care. The ones that didn’t work helped us learn what needed to be improved in our AI tools. We think this research opens the door to new ways of thinking about antibiotics and drug discovery, and this first step will allow scientists to explore it with increasing creativity and precision.”
Read the full story in Penn Engineering Today.