2025 Team PRIME – Engineering a Smarter Response to Sepsis

The final feature in the 2025 Senior Design Awards Spotlight highlights Team PRIME, who earned Second Place at the Minnesota Design of Medical Devices Competition.
Team Members: Majd Ayyad, Shriya Boyapati, McKenzie Davis, Sophie Gu, Sophie Klessel
Senior design in Penn Bioengineering is a yearlong capstone experience in which bioengineering seniors identify an unmet bioengineering need, design a solution to address the need, and create a high quality prototype that demonstrates their design. The course consists of BE4950 and BE4960, and was most recently taught by Dr. Erin Berlew, Dr. David Meaney, and Dr. Michael Siedlik.
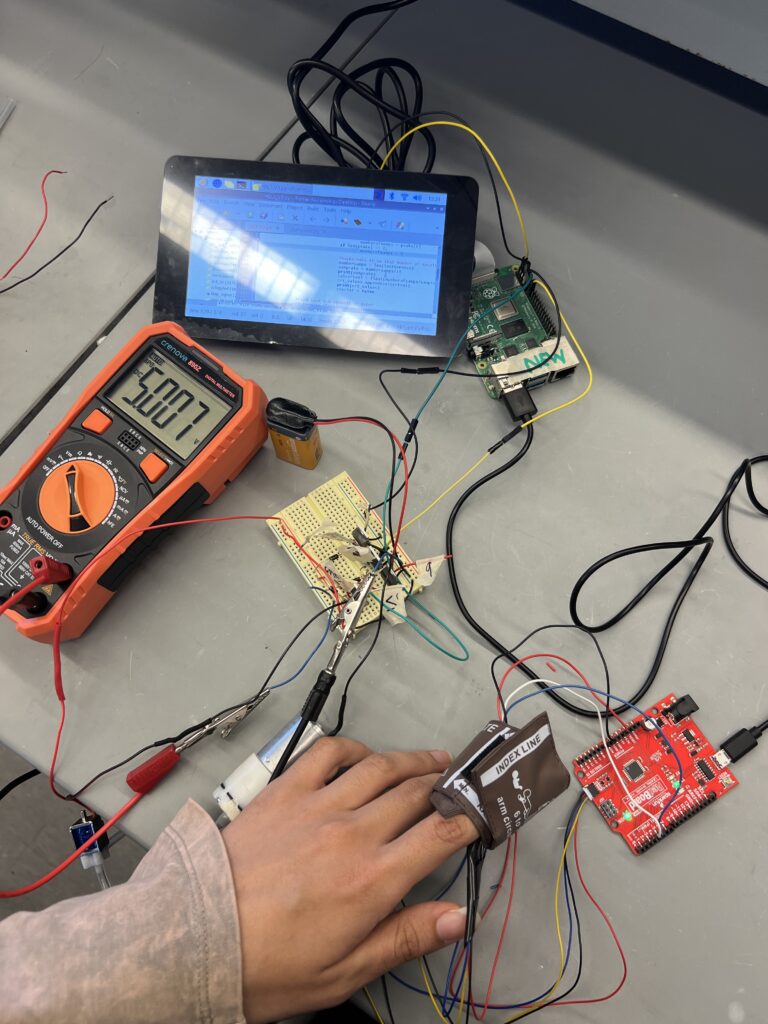
For Team PRIME, the mission was clear: create a tool that could help detect sepsis earlier—when timing can mean the difference between life and death. Their project centers around a device that automatically measures capillary refill time, a simple but powerful indicator of blood perfusion and circulation quality. By providing continuous, automated monitoring, PRIME aims to improve clinical decision-making in intensive care units and emergency settings.
The team’s inspiration came from their clinical mentor, Dr. John Greenwood, whose passion for improving sepsis detection was contagious.
Sophie Klessel shared, “We had a great clinical mentor (Dr. John Greenwood) who was really passionate about creating a device for earlier detection of sepsis, and we knew we wanted to work with him. Additionally, sepsis was an issue that resonated with our group and an issue that we were excited about.”
Team PRIME approached the work with a strong sense of collaboration, blending individual strengths across software, hardware, and systems integration. One member led the development of the user interface and application logic, while another focused on designing and assembling the physical and electrical components.
Working on PRIME revealed to the team just how demanding and rewarding bioengineering can be.
“Bioengineers need to understand it all from interviewing clinicians for needs findings, to studying the physiology of the human body, to designing all the technical components including hardware and software and finally towards producing a medical device. It is such a difficult job to be all the engineers at once but the final results are rewarding!” Majd Ayyad explained.
As the project concluded, their work was already gaining traction. Dr. Michael Siedlik, one of the bioengineering senior design instructors, shares, “This technology could greatly surpass the current standard of care, as it provides much needed automation, reproducibility, and clinician-free measurements in hectic medical environments where quick and reliable measurements are critical for preventing the negative outcomes of sepsis.”

PRIME earned Second Place at the Minnesota Design of Medical Devices Competition, a national recognition of the team’s thoughtful engineering and strong clinical relevance. Development of the device will continue in partnership with their clinical mentor—bringing them one step closer to impacting real patient care.