To commemorate the 50th anniversary of the Department of Bioengineering at the University of Pennsylvania, the department has acquired several pieces of artwork that celebrate the beauty of biological forms. The pieces were curated by Nicole Lampl, Director/Curator of the Reeves House Visual Arts Center.
Read a message from Department Chair Dr. Ravi Radhakrishnan: “Penn Bioengineering: The Past, Present and Future“

Vertex (2019)
Artist: Betty Busby
Fiber, 66″ W x 56″ H
Created with a limited palette on artist dyed silk and hemp, Vertex makes a strong impression of motion in the branching imagery derived from fractals.
“I went to the fractal show at the New Mexico Museum of Natural History & Science’s planetarium, and it blew my mind,” says Busby. “They go from a picture of the galaxy down to a picture of an atom, and you see the same image repeated again and again.” The artist’s focus on macro imagery is the product of her lifelong fascination with molecular biology. Constantly exploring new materials and techniques from around the world, Busby has purchased batiks from Bali, dupioni from India, and silk from China that she paints and dyes with acid. The artist sees the variety of materials that she used in her mixed media works as a direct reflection of the incredible diversity found among living things.
About Betty Busby: After graduating from the Rhode Island School of Design with a BFA in ceramics, Betty Busby founded a custom ceramic tile manufacturing firm in Los Angeles. After nearly 20 years of running the firm, she sold the business in 1994 (it is still in operation to this day). Upon relocating to New Mexico, she changed the focus of her artwork to fiber, taking it full time in 2004. Her manufacturing background has lead to constant experimentation with new materials and techniques that fuel her work. Originally inspired by Amish quilts at the Kutztown County Fair near her childhood home in Pennsylvania, her work has made the journey from bed quilts to mixed media sculpture, and is constantly evolving and heading in new directions.
Artist Statement: Betty Busby creates fiber art using technological innovations and unconventional materials to create work with inviting textures. She is often inspired by the macro world, exploring the structures and forms of nature. She uses these images as jumping off points to create abstractions, which become ground-breaking works of art. Betty Busby creates fiber art using technological innovations and unconventional materials to create work with inviting texture. But the voice of textile roots is strong with traditional fabric, paints and dyes, needle and thread and her trusty Singer working alongside her iPad and spun bonded nonwoven fibers.
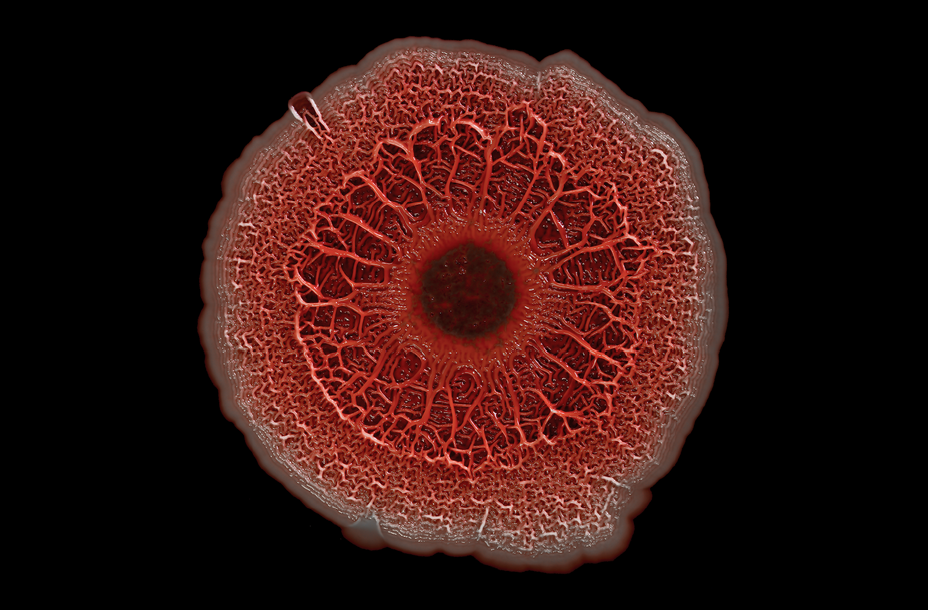
Pseudomonas Aeruginosa Colony Biofilm (2023)
Artist: Scott Chimileski
Photography mounted on board, 24″ W x 16″ H
The most harmful species of microbes build biofilms and swarm together. When the conditions are right, the Pseudomonas Aeruginosa (pictured here), can shift from a harmless bacterium found in many environments to a pathogen that causes infection in burn wounds.
About Scott Chimileski: Scott Chimileski a microbiologist, imaging specialist, and educator based in Woods Hole, MA, where he is a Research Scientist at the Marine Biological Laboratory (MBL). From 2015 to 2019, he was a postdoctoral fellow in the Kolter Lab within the Department of Microbiology at Harvard Medical School. During that time, Roberto Kolter and Chimileski curated the exhibition Microbial Life: A Universe at the Edge of Sight, open at the Harvard Museum of Natural History from February 2018 through March 2022. They also coauthored Life at the Edge of Sight: A Photographic Exploration of the Microbial World, published by Harvard University Press in 2017. Chimileski’s imagery has been published or broadcast by media outlets including National Geographic, WIRED, TIME, The Atlantic, STAT, Fast Company, NPR, The Scientist, Scientific American, Smithsonian Magazine, The Biologist, HHMI Biointeractive, Tangled Bank Studios, Quanta Magazine, the NIH Director’s Blog, WBUR Boston, The Verge, TED Talks, and CBS Sunday Morning. Exhibitions at public venues across the United States, and in Uruguay, Brazil, Colombia, Scotland, the UK, and Denmark have featured his imagery and scientific interpretation. Chimileski received a Passion in Science Award in Arts & Creativity from New England Biolabs in 2016, and FASEB BioArt awards in 2016, 2017, and 2019.
Artist Statement: Chimileski’s original scientific photography specializes in high resolution macrophotography and time lapse imaging of microbial colonies and behaviors. This collection includes photos captured at sites around the world where exceptional natural microbial forms flourish, such as Yellowstone National Park. Most bacterial and archaeal cells are far too small to see with the naked eye. However, microbes are seldom if ever found in isolation. Rather, the biology of the microbial world is underpinned by the tremendous interactivity, sociality and modularity of individual cells, which often coalesce in great numbers to produce macroscopically visible structures, including biofilms, microbial mats, colonies, swarms and fruiting bodies. Chimileski is focused on the development of macroscopic imaging techniques as well as time-lapse photography and three-dimensional scanning technologies as applied to microbial multicellular forms, collective behaviors, communities and interspecies interactions. He is also interested in leveraging the power of photography as a medium for communicating microbiology to other scientists and to the general public.
Amoeba Hex Pod (2018), Amoeba (2013) and Amoeba Coffin (2013)
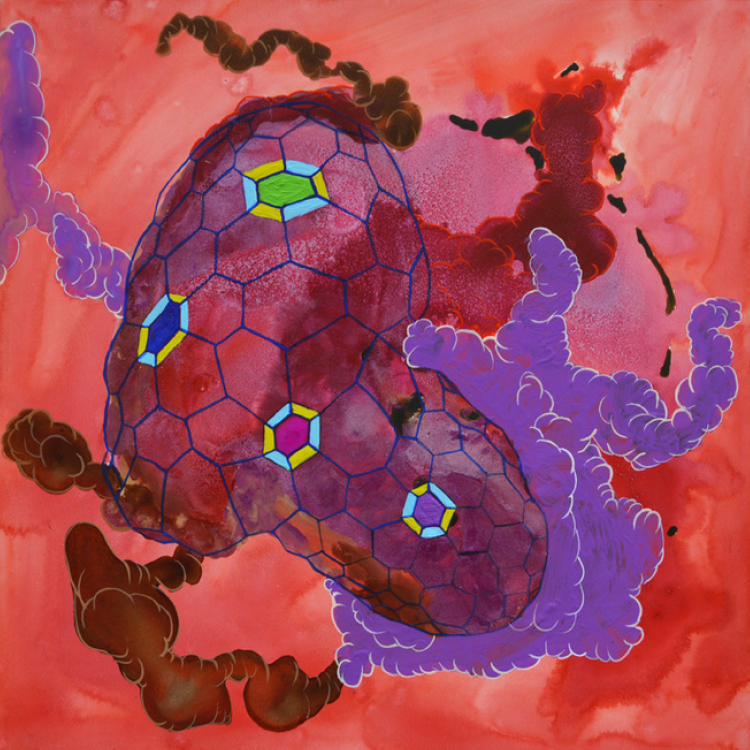


Artist: Melissa Bolger
Gouache, ink, and graphite on clayboard, 6″ W x 6″ H x 2″ D
Bolger explores Synthetic Biology and the myriad ways in which it can imbue engineered organisms with new abilities. Redesigned and entirely imagined cellular structures coexist and intermingle as the artist investigates an unseen universe. Through her visual exploration of this scientific field, the artist invites us to ponder what the consequences of replicating nature on a cellular level might have on human evolution.
About Melissa Bolger: Melissa Bolger is a California native and was raised outside of Redding, CA where her parents settled on a remote piece of property, built a house, and raised their family off the grid. her mother sewed the family’s clothes and other household items. For Bolger, the woods were her playground and she grew up hiking, fishing, hunting, riding horses and panning for gold. Some of her early artistic influences grew from those days, living off a dirt road overlooking a canyon and creek, when do-it-yourself was the only way to get things done. Today, she merges the techniques of craft with fine art in her interpretative portraits, recycled materials, paintings and drawings. Melissa Bolger’s work has been exhibited in solo and group shows and her work has been reviewed in publications.
Artist Statement: The “Soft Machines” series explores themes of patterns within nature through the intricate application of pen and ink, gouache, and graphite. Her interest is on cellular structures that are manipulated by synthetic and artificial life. Borrowing from nature and science, microscopic shapes and images are drawn and high-key colors painted that float, hover, and drip in visual metaphors that insinuate synthetic manipulation. Patterns of nature are complex on a nanoscale and certain thoughts arise. What would be the consequences of science’s attempt to replicate nature on a cellular level? How far will synthetic operations continue in human history? What effects will they have on evolution? The manipulation of nature at the nanoscopic level is overwhelming, mind-blowing and psychedelic. While this manipulation has the potential to alter human life in numerous uncharted ways the question of how and what form life will survive in a synthetic and artificial way is mysterious, puzzling and hi-tech. Approaching these themes with curiosity and instinct, exploring and documenting the natural and the unnatural together and maintaining a sense of wonderment is the embodiment of “Soft Machines.” Examining the intricacies of the invisible world give birth to patterns that move like a heartbeat, live and survive against all odds. “Soft Machines” is the beginning of a series of work exploring, investigating and examining particular themes around astrobiology, synthetic cellular and molecular reconstruction. Bolger continues to explore themes of patterns within nature on a nanoscopic scale in her intricate application of pen and ink, gouache, graphite and mixed media. The invisible world under a microscope is a fascinating phenomenon that Bolger uses as a stepping point into inner realms of space that move, float, and drip. Whether it be an alien landscape or intricate organic patterns, the diversity of life on the planet is an essential force and fascination within the work.
Links:
Betty Busby:
Website: bbusbyarts.com
Instagram: @bbusbyarts
Scott Chimileski:
Website: scottchimileskiphotography.com/
Instagram: @socialmicrobes
Melissa Bolger:
Website: melissalouisebolger.com
Instagram: @melissalouisebolger
Nicole Lampl
Website: nicolelampl.crevado.com
Instagram: @thecuriouscurator_nicole
Email: njlampl@gmail.com
Phone: 504-428-8589