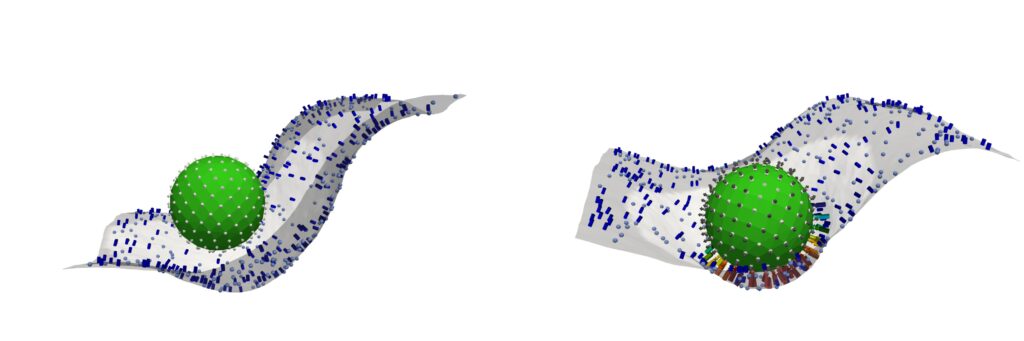
Penn-led researchers have turned a deadly fungus into a potent cancer-fighting compound. After isolating a new class of molecules from Aspergillus flavus, a toxic crop fungus linked to deaths in the excavations of ancient tombs, the researchers modified the chemicals and tested them against leukemia cells. The result? A promising cancer-killing compound that rivals FDA-approved drugs and opens up new frontiers in the discovery of more fungal medicines.
“Fungi gave us penicillin,” says Sherry Gao, Presidential Penn Compact Associate Professor in Chemical and Biomolecular Engineering (CBE) and in Bioengineering (BE) and senior author of a new paper in Nature Chemical Biology on the findings. “These results show that many more medicines derived from natural products remain to be found.”
From Curse to Cure
A. flavus, named for its yellow spores, has long been a microbial villain. After archaeologists opened King Tutankhamun’s tomb in the 1920s, a series of untimely deaths among the excavation team fueled rumors of a pharaoh’s curse. Decades later, doctors theorized that fungal spores, dormant for millennia, could have played a role.
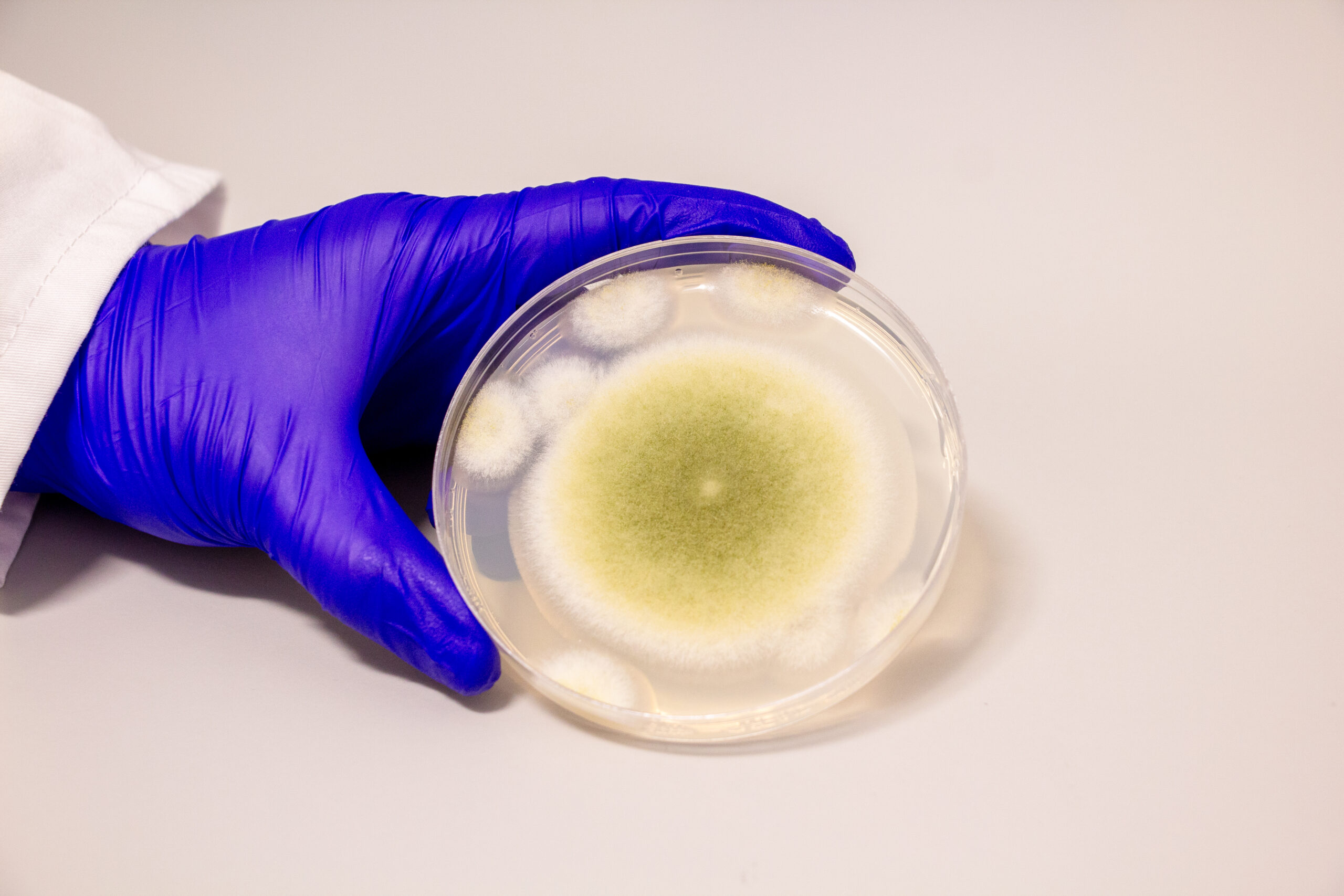
In the 1970s, a dozen scientists entered the tomb of Casimir IV in Poland. Within weeks, 10 of them died. Later investigations revealed the tomb contained A. flavus, whose toxins can lead to lung infections, especially in people with compromised immune systems.
Now, that same fungus is the unlikely source of a promising new cancer therapy.
Read the full story in Penn Engineering Today.